Candida yeasts — the most common of which is Candida albicans — normally live in and on the body, without causing any symptoms. However, Candida can overgrow, and cause “thrush” and “yeast infections”. In addition, it can enter the bloodstream and cause candidemia — althougha extremely rare in people without risk factors, candidemia is the fourth most common bloodstream infection among hospitalized patients in the United States.
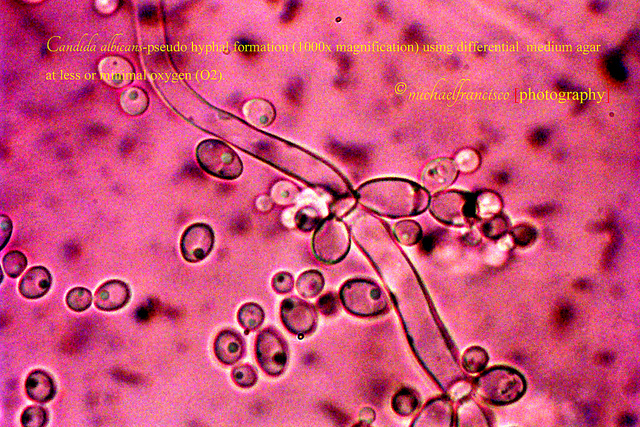
Until very recently, scientists thought that Candida albicans spread by changing shape — from a single, round cell, to a long string of cells, or filaments. The prevalent thought was that, by changing shape, the fungus could move through the bloodstream, let its filaments penetrate tissues, and destroy a specific type of immune cells by inducing an inflammatory programmed cell death. This specific type of immune cells, or macrophages, are the same immune cells that would otherwise kill the fungus. Instead, the fungus filaments induces macrophage pyroptosis.
A wide variety of infectious microorganisms cause the death of their host cells, and they do so using different mechanisms. Pyroptosis is one of the three forms of cell death (the other two are apoptosis and necrosis) — it’s a programmed cell death associated with the antimicrobial responses that macrophages mount during inflammation. In this process, macrophages recognize certain danger signals within themselves and — in response to these signals — produce cytokines, swell, burst, and die.
However, results from a new study show not only that macrophage death is triggered by a little bit of sugar that appears on the surface of fungal cells, but also that the sugar appears only when the fungus is inside the macrophage. Leah Cowen, lead study researcher, said in a press release: “It’s not the shape-change per se that enables the fungus to kill the immune cell, but what happens along with it. The addition of glycosylated proteins, which are proteins with a sugar attached, remodels the surface of the fungal cells.”
Macrophages engulf invading infectious microorganisms, including Candida albicans. Following engulfment, the invaders wind up enclosed in phagosomes — vescicles that contain the invaders, and create an environment in which the invaders can be destroyed.
The researchers found that Candida albicans remodels its surface in response to the environment it encounters in the phagosome — the remodeling of the fungal cells induced macrophage lysis (swelling and bursting). Induction of macrophage lysis occurred even if Candida albicans is dead. In other words, ingestion by macrophages causes the fungus to change its surface through addition of glycosylated proteins — the resulting altered fungus surface is sufficient to induce macrophage lysis in the absence of C. albicans growth.
Researchers used an enzyme called Endo H to snip off sugars on the glycosylated proteins attached to the dead fungal cells. Removal of the sugars completely blocked the ability of the fungus to kill. This finding provides a strong lead on a new and needed therapeutic strategy for Candida albicans — thus, such new therapeutic strategy could be based on agents that remove sugars from the altered surface of the fungus.

Some pathogens, such as the mycobacteria that caused tuberculosis, thrive inside macrophage. They do so by inhibiting the macrophage from joining the phagosome with the lysosome–which would normally cause the death of the pathogen. The mycobacteria is able to avoid other immune cells, while remaining safe inside the macrophage. CD4+ T cell 1 overcomes this by activating the macrophage to join the phagosome and lysosome. CD4+ T cell 1 will attach to the CD40 receptor on the macrophage and releases IFN-gamma cytokines to initiate activation.
Reading this article makes me wonder why the CD4+ T cells are not activating the macrophage to destroy the fungus before pyroptosis begins?
That’s a great question, at first I thought that the macrophages die before they have a chance to send out the cytokines that would activate the CD4+ cells. But in the article it stated that they do in fact produce the cytokines before they die. I think that the CD4+ T cells are not activated because the macrophages begin to swell up as soon as the fungi enters the phagosomes and changes it’s surface. In the case with the mycobacteria that caused tuberculosis, there is no immediate harm to the macrophage, and there is enough time for CD4+ T cells to come to the rescue and allows the phagosome and lysosome join.
Here is a very simple example that helped me understand the difference:
The mycobacteria that caused tuberculosis are like un-armed teenager shop lifters in a gas station. Once inside the gas station, the store clerk can lock the door (phagocytize) and contain the shop lifters until the police (CD4+ T cells) can come and help get rid of the problem. But with the fungi, they’re more like armed terrorist, as soon as they come into the gas station, they shoot the store clerk.
Little confusing example but I hope it helped.
That’s a pretty good analogy. But it still leaves a couple questions unanswered. Since the macrophages burst, it gives the impression that the content are released as well (unlike apoptosis where the cell shrivels and dies). The fact that the content of the macrophage are now out in the system should trigger a “danger” signal since you don’t expect a nucleus or an endoplastic reticulum floating around in connective tissue. I would assume dendritic cells could take these in an process them to activate CD8+ T cells to attack infected macrophages and maybe even the fungi themselves. Now this is speculation based of similar cancer treatment where a cancer is lysed and it’s contents are processed and presented by DC to CD8+. If the internal structures are released from a macrophage that has burst, my question would be why wouldn’t a similar response cascade not be triggered.
It’s like if the clerk where shot released his internal organs and the police was able to use his organs to find the bullet and use it to track the terrorist and kill them.
I don’t think a a similar response cascade can not be triggered because when the macrophages burst, they do not send a distress signal, which I would assume to be a cytokine. In the paper, it does say that the macrophages send out cytokines, but it does not specify what type of cytokine or what purpose the cytokine has. Going back to the analogy, the store clerk can take his time and call the police (release specific cytokines) when the teenage shop lifters are there, but with the fungi, before the store clerk has the time to contact the police, he’s shot. Furthermore, I think the reason why the “internal organs” do not send a distress signal is because maybe the new structure of the fungi keeps it from being detected. To add even more to the analogy, the terrorist are in normal clothes, they come in the gas station, put a disguise on, kill the clerk, and once they go outside no one knows who they are because they have a disguise.
That fact that the fungi can be compared to terrorists with disguises is truly terrifying. It stills seem that it should be possible after several similar occurrences for the cell to recognize the signs of an oncoming attack. The fact that no witnesses and no evidence is left behind is what really makes this interesting. I wonder what therapeutic strategy concerning the sugars on the glycosylated proteins will come to fruition. Hopefully a viable solution comes forth soon.
You misunderstand the distress signal concept I am trying to convey. Cellular components such as the endoplastic reticulum, nucleus, mitochondria, etc do not belong free floating in the connective tissue or blood. When such components are picked up by antigen presenting cells (APCs), it alerts them of a ‘danger’ since in normal circumstances they would not find those components. It is a hallmark of cancer therapy treatments as radiation therapy destroys cells and those tumor components are picked up to be presented to prime the adaptive immune system to the specific tumor. We aren’t talking about cytokines here like the original post mentions, but actual cellular components. If it works for tumor therapy, why wouldn’t the same work when the macrophage bursts. You still have dendritic cells and B cells that could act as APCs. Unless the fungi destroy the cellular components, which is not happening here, there would still be something left behind for the APCs to pick up.
So my original analogy still stands. The internal organs, or even the body, left behind by the terrorists allow the police (in this case APCs) to use the bullet in the body to track it to the gun and hence find the terrorists to kill them. Despite being in normal clothes, anything biological has a specific set of receptors or distinguishing characteristic that can be used by APCs to present to the adaptive immune system. So thanks to the specific fingerprint, the police are able to find the terrorists.
I wonder if it is possible that the ingestion of the fungus causes different cytokines to be released. Even though the fungus causes the macrophage to lyse, it has been pointed out that cytokines are still released. The fungus changes the surface of the phagosome, so maybe this change can cause other cytokines to be produced by the macrophage. There are many different types of cytokines, so it would be interesting to test the types of cytokines that are produced when a cell encounters the fungal infection. This could explain why the CD4+ T cells are not activating the macrophage before it undergoes pyroptosis.
It would be interesting to test for a change in cytokine production when the fungus is ingested by the macrophage, however the phagosome surface is not changed by the fungus; the fungus changes its own surface shape inside the phagosome. So perhaps the lack of recognition of the cytokines by CD4 T cells is due to a potential prion-like reproduction habit exhibited by the fungus and not through interactions of the macrophage and the phagosome. In terms of therapeutic options though, would an alteration of the environment created by the phagosome also inhibit the fungus from inducing cell death? With or without cleaving the sugars?
I am quite certain that ingestion of the fungus by the macrophage causes the release of several different types of cytokines as in the case of Mycobacterium tuberculosis, such as: IL-1β, TNF-α, IL-6, CXCL8, and IL-12 to name a few. Candida albicans is a commensal organism that is a part of the normal microbial flora associated with mucous surfaces in the human body. Infections occur due to overgrowth of this normal flora. Keen reading of the article explains this concept. Furthermore, it is not the surface of the macrophage that is first change by the bacteria, but the “remodeling” of the surface of C. albicans in the phagosome.
It is important to understand that cells of the immune system have cell surface receptors that aid cell in pathogen recognition. For instance, macrophages use pattern recognition receptor to bind fungal components. Figure 3.4 of chapter 3 of The Immune System, Illustrates the receptors on the macrophages that are able to recognize carbohydrates are called lectins. Mannose receptors and dectin-1 are two example of receptors present on the macrophage among many others. However, for fungal recognition of Candida albicans, these receptors are important to mention. According to an article Immune Responses to Fungal Pathogens illustrate that it is the CD4+ T cells that is activated during an adaptive immune response to the fungi.
According to Parham, T cells function by making contact with other cells like macrophages in an innate immune response and B cell during an adaptive immune response. It would be necessary for an activated innate immune response with the macrophage exercising its phagocytic prowess, inhibiting fungal cell growth. This would be necessary for the activation of CD4+ TH1 cells. Destruction of the macrophages inhibits antigen presentation on MHC class II molecules to the CD4+ T cell, which in turn cannot increase the phagocytic properties of macrophages. Therefore, the innate immune response need to recruit a more effective killer, possibly the neutrophils and even dendritic cells.
I wonder if it is possible that the ingestion of the fungus causes different cytokines to be released. Even though the fungus causes the macrophage to lyse, it has been pointed out that cytokines are still released. The fungus changes the surface of the phagosome, so maybe this change can cause other cytokines to be produced by the macrophage. There are many different types of cytokines, so it would be interesting to test the types of cytokines that are produced when a cell encounters the fungal infection. This could explain why the CD4+ T cells are not activating the macrophage before it undergoes pyroptosis.
Well, it is known that viruses such as influenza virus change their form so that they are not detected rapidly by the immune system since no memory cells are generated. As for fungus, it appears that they tend to change their morphological structure based upon being in a certain environment. So when they are engulfed by resident cells macrophages, they alter their shape and express glycoproteins. Usually macrophages provide a constant, long lasting defense mechanism against infectious agents since they are large cells that contain vacuoles, which are well equipped for phagocytosis. As observed, Candida albicans can protect themselves while in the phagosome vesicle by producing the sugar substances on their surface. When macrophages are destroyed via pryoptosis, they will lose the ability of releasing cytokines that signals for the recruitment of other immune cell, thus leading to a weakened immune system. The interaction between the Candida albicans and macrophage has been studied and the outcomes showed that the C.albicans destroy macrophages in two phases that occur upon altering the form of fungus. Phase 1 involves the piercing of the macrophage plasma membrane while the second phase involves the activation of pryoptosis of the macrophage. Thus, Endo H enzyme would prevent the progression of the two phases used by C.albicans, therefore, fungus would not be able to escape the phagocytosis process.
In the study it was shown that filamentation was not required to lyse the macrophage and also was not the likely cause of pyroptosis. This was done by repressing gene the genes responsible for filamentation, but still observing lysis of macrophages that had engulfed the fungus.
This finding is pretty interesting. I think that antifungal medications that inhibit or disrupt ergosterol synthesis are the more efficient treatment. However, a treatment using the glycosidase Endo H could prove to be of therapeutic value to patients that are immunocompromised. Especially those that are in the early stage of HIV infection.
These findings made me do some research in the gynecologic area. The infamously common vaginal yeast infections are caused by Candida albicans, and one symptom is white and thick discharge. It is known that the macrophages secrete cytokines such as IF-1B (Krysan 2014) and CXCL8, which induce and act in the inflammatory response. During the response, neutrophils are recruited abundantly and die by apoptosis quickly. The dead neutrophils are the discharge. However, in this case, macrophages are being readily lysed, so they may add to the thickness of the discharge of vaginal yeast infections.
Krysan, D. J., Sutterwala, F. S., & Wellington, M. (2014). Catching Fire: Candida albicans, Macrophages, and Pyroptosis. PLoS Pathogens, 10(6), e1004139. doi:10.1371/journal.ppat.1004139
I am wondering why or if it’s a problem that the macrophage dies in response to the fungal infection? This is a functional response that the cells use when infected. The macrophages are the conductor cells of the immune system where they detect and signal when an infection is detected. It was said the macrophages are able to produce cytokines before they die, so where are the neutrophils? And there are monocytes circulating waiting for the signal to produce more macrophages. So now I am wondering, as stated by someone else, are the fungal contents released upon the lyses of the macrophage? Macrophages are already responsible for getting rid of the dead neutrophils once they do their job so I’m assuming the macrophages get rid of these dead cells too. So when the infected macrophage lyses, if the contents are released, is it an on going cycle and that’s where the problem lies?
I believe the problem lies within the fact that once the macrophage lyses, it can no longer breakdown the fungus and it does indeed release all contents into the body. The cytokines released are not inducing recognition by other cells which allows the fungus to continue to grow without regulation by the immune system. I’m not sure if there would be an indefinite ongoing cycle, but perhaps the cycle would be continued until all the macrophages were lysed?
So through the use of a single sugar the Candida Yeast keeps themselves from being destroyed by the macrophages. They are pretty much acting as cytokine bombs flowing around the bloodstream when they have infected a person. So because they cause a cytokine release before they make the macrophage lyse, is this leading to a cytokine storm? Also would they be considered endotoxic like bacteria because even after death the sugars on their membrane are causing the macrophages to swell and burst. Which seems similar to that of endotoxic bacteria that cause even more damage once they die and start breaking down.
Well I had no idea that candida could enter the blood stream. The microbes that bring us bread, beer and other goodies, can actually go past the surface of the skin. I suppose they did say it was rare to anyone without risk. Being the fourth most prominent bloodstream infection in U.S. hospitals. , its easy to see why this research was funded and founded. It’ll be interesting to see what vector the produce around Endo H to weaponize it against yeast infections. Endo H cleaves sugars on the yeast surface. That’s fantastic, but yeast are a little closer in relation to our cells than bacteria, being eukaryotic. There’s most likely years of testing ahead for whichever drug company picks this up, just to make sure there’s no negative side effects.