A few months ago, Zmapp made its way across the ocean — from the United States to Liberia, where it was administered to Kent Brantly and Nancy Writebol. Both of them had contracted Ebola while doing missionary aid work, helping people infected with the virus. Both of them recovered. However, we don’t know the exact role that Zmapp — an experimental drug never before tested on humans — played in their recovery.
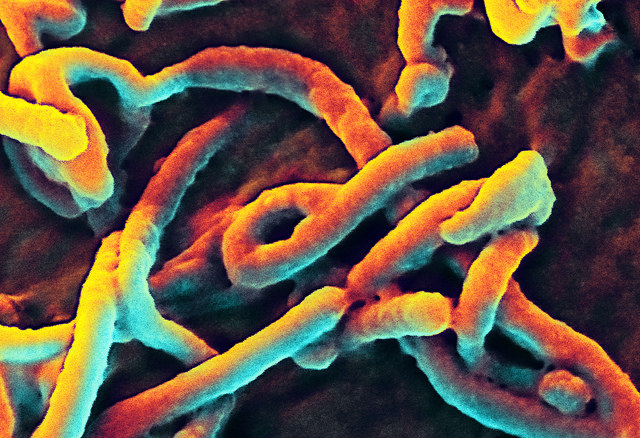
Dina Fine Maron (Nature/Scientific American) asked Bruce Ribner about Zmapp. Ribner is the medical director of the Infectious Disease Unit at Emory University Hospital in Atlanta, Georgia, where both Brantly and Writebol were treated after being flown there from Liberia.
He said: “Experimental drugs are experimental drugs because we don’t know if they will work. That is true both with the preparations patients received in Liberia and other preparations that are being considered for treating patients with this infectious disease. We are a long way from being able to say that someone that received one of these agents benefited, it had no impact or it may be that their outcome may be impeded. Until we have good studies looking at outcomes of patients who received these medications, compared to patients who didn’t receive them, we should be very cautious.”
Zmapp is a combination (also called a cocktail) of monoclonal antibodies developed by Mapp Biopharmaceutical, a company based in San Diego. The drug showed efficacy in recent studies conducted in a monkey model by the Public Health Agency of Canada. It is manufactured in Kentucky by the company Kentucky BioProcessing (KBP) using fast-growing, low-nicotine tobacco plants, which are grown indoor under tightly controlled conditions. The tobacco plants act as “photocopiers” that produce large amounts of monoclonal antibodies — these are then extracted from the plants and processed into the drug,
Due to the present Ebola epidemic, Mapp and KBP are working with the U.S. government to accelerate scaled up production of Zmapp. Until now, these companies were producing only amounts sufficient for safety and efficacy testing in animals. In the mean time, researchers are studying how Zmapp combats Ebola. A new study (Structures of protective antibodies reveal sites of vulnerability on Ebola virus) published on December 2, 2014, in the scientific journal Proceedings of the National Academy of Sciences, describes weak spots on the surface of the Ebola virus. These weak spots are those targeted by the monoclonal antibodies present in ZMapp.
Using a special type of electron microscopy, the researchers found that two of the ZMapp antibodies bind near the base of the virus, appearing to prevent the virus from entering cells — a mechanism called “neutralization”. A third antibody binds near the top of the virus, possibly acting as a beacon to call the body’s immune system to the site of infection.
Andrew Ward, one of the lead authors of the study, said in a press release: “Now that we know how ZMapp targets Ebola, we can compare all newly discovered anti-Ebola antibodies as we try to formulate an even better immunotherapeutic cocktail.”
ZMapp is expected to go into human clinical trials in early 2015.
Copyright © 2014 Immunity Tales.

Until recently, neutralizing antibodies were unknown but with technology, molecular characterizations of antibodies have given hope in order to discover new vaccines or drugs, for example Zmapp. The new study that the blog mentions ( Structures of protective antibodies reveal sites of vulnerability on Ebola virus ) found how monoclonal antibodies against the Ebola virus each bind one of three distinct regions. These regions, therefore, constitute at least three sites of vulnerability on the surface of this virus. Furthermore, other studies ( Broadly Neutralizing Antibodies Present New Prospects to Counter Highly Antigenically Diverse Viruses ) have found the same consistent sites of vulnerability but in other viruses such as HIV-1 and influenza.
The first study have also mentioned that some of the anti-ebolavirus antibodies are neutralizing. As we know, neutralization process by antibodies is crucial because they have the capacity of neutralize the biological effects of the antigen. A study that used HIV-1 and influenza hemagglutinin (HA) as glycoproteins ( Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA ) mentions that neutralizing antibodies focus their recognition on the similar sites like the receptor binding ,the membrane fusion machinery, and quaternary epitopes in both viruses. This fact highlights the new study by Murin et al. because they indeed found the exact locations where neutralizing antibodies bind to the antigen. Overall, we could say that in a way HIV-1 and influenza virus studies have helped investigate in depth how exactly these monoclonal antibodies bind to Ebola virus.
According to WHO
the Ebola virus first appeared simultaneously in 2 outbreaks in 1976 coming from Sudan and the Democratic Republic of Congo. However, it was fairly contained and didn’t spread to the Western hemisphere until 2014. It wasn’t until recently that an experimental drug, Zmapp, was produced to combat the virus. It is still unknown exactly how the drug works but researchers from the study Structures of protective antibodies reveal sites of vulnerability on Ebola virus
, provided the structure of every monoclonal antibody in the ZMapp cocktail. ZMapp is a combination of both MB-003 and ZMab. What the researchers did was identified the binding sites of antibodies in both MB-003 and Zmab to get a better understanding of how Zmapp binds. MB-003 contains antibody c13C6, c13F6, and c6D8 while ZMab contains c1H3, c2G4, and c4G7. ZMapp itself contains c13C6, c2G4, and c4G7 which means ZMapp is a hybrid of the cocktails. Researchers found that c13C6 (from Zmapp) and c1H3 (from Zmab) binds within the glycan cap of the virus while c2G4 and c4G7 binds to Ebola GP ectodomain. The antibodies bind to the three vulnerable sites on the virus’s surface, 2 mucin domains and the glycan cap. The binding of antibodies to the 3 vulnerable sites of the virus surface is what differentiate ZMapp from previous cocktails. However, there’s still more research to be done on both ZMapp and the Ebola virus. Even though both Dr. Kent Brantly and Nancy Writebol were given the drug and survived, Rev. Miguel Pajares was also given the drug and died. There may be a certain time span that the drug needs to be given or something different but right now, ZMapp remains an experimental drug until it is better understood.
Hopefully the human clinical trials of Zmapp shows positive promise due to its previous successes this year with patients who contracted Ebola. A successful therapy method against Ebola would dramatically slow down the spread of the disease and save countless lives in the process. The question I think is important to look at, is how far can future Zmapp cocktails not only treat already infected patients but also act as a preventive tool for future outbreaks. According to the blog post, the Zmapp treatment is made out of a cocktail of monoclonal antibodies that are created from tobacco plants that are kept in an isolated controlled environment. This cocktail of antibodies would be then given to an infected patients who at that moment does not have any antibodies for the disease, especially for the populations that are not geographically located near the outbreak. The idea would be that these antibodies would hold the disease back long enough for the patient to produce its own antibodies and fight off the infection.
However, would these patients who have been treated with Zmapp have an immunity towards Ebola? If your own body successfully produced its own antibodies against the Ebola virus, it would also have made memory b-cells for those antibodies which can be produced faster and show higher affinity to the antigens of Ebola. Yet the Ebola virus works very rapidly, according to Fighting Ebola with ZMapp: spotlight on plant-made antibody, it can cause death after six to nine days after the initial symptoms appear. Would six to nine days be enough for your own antibodies to counter the virus or would its rapid multiplication outpace the immune system. It would be interesting to see if Zmapp could be turned into a booster shot for people who travel in areas where the outbreak of Ebola is possible. This would be something to think about with the emergence of vaccines for Ebola, and if they alone are enough to prevent the infection of the disease.
This is a very important point. Zmapp, as well as other drugs containing monoclonal antibodies, act exactly in this way. They keep the infectious agent at bay until the immune response kicks in and starts producing effective antibodies (or other immune components such as T cells specific for the virus). As you said, this results also in memory responses and, as said around the news, people that survive Ebola are protected from further infection at least for sometime. Zmapp was used in patients infected with Ebola exactly because the expectation was that the monoclonal antibodies in it would keep the virus in check until the infected people started making their own antibodies.
I think that it is great that scientists have discovered the Ebola vulnerability sites that the vaccine Zmapp can bind to, but how long do you think that just targeting these sites would be an effective method of combating this virus? It is common knowledge that Ebola is a highly lethal and dangerous virus so how long do we think this will be effective before Ebola decides to mutate and change its structure to inhibit the vaccine. Zmapp appears to be a very specific vaccine in that it is made specifically for certain sites on the virus and ultimately, a decent modification in one of the vulnerability sites would render them useless. Eventually the Ebola virus will get smart and change its structure as often seen in other problematic viruses such as HIV. I think it would be important to potentially identify other sites of vulnerability. In the paper, Ebolavirus glycoprotein structure mechanism and entry, researchers Lee and Saphire show that the EBOV glycoprotein (GP) is very critical to viral attachment and the importance of vaccines targeting this protein. This could also serve as another option for those who may have other issues that also contribute to the effectiveness of their body getting rid of the Ebola virus. These issues include factors such as timing, lifestyle, health, age, race and sex. I do not think it’s fair to say that by developing a vaccine to attach its monoclonal antibodies to the vulnerability sites that this would effectively eradicate this disease. After all, out of the three patients who have been given the experimental drug Zmapp, only two survived so to me that would indicate that other factors need to be taken into consideration.
Onooks, you made a very good point here. Since Ebola is a highly mutating virus, it is only a matter of time before ZMapp antibodies are no longer effective against infected cells. Further studying points of vulnerability in the Ebola virus will help researchers find stronger vaccines that work similarly to ZMapp. In a similar article posted last month, “the sites where the ZMapp antibodies bind to Ebola have so far been unaffected by the more than 300 genetic changes identified in the current strain of the virus” (http://www.xconomy.com/san-diego/2014/11/17/new-study-shows-how-zmapp-binds-to-ebola-ways-to-boost-potency/) . The genetic changes in the virus do not prevent ZMapp from attaching to the Ebola virus; the replication of the virus will continue until the necessary antibodies are made to combat the constantly evolving Ebola virus. You also mentioned how attaching monoclonal antibodies to vulnerability sites would not eradicate the disease. I agree that Ebola wouldn’t be eradicated this way, but it could drastically reduce the infection rate of the virus. Although eradicating the disease would be the best for this current epidemic, further developing ZMapp will help provide (short-term) protection against current strains of the Ebola virus.
With the recent unexpected up rise in the Ebola virus disease (EVD) , a great deal of time, attention, research, and resources have been put towards finding a cure to this epidemic. In this day and age, travel has become incredibly efficient—both humans and fatal pathogens alike capable of traversing most parts of the world in less than a day. With its ease of transmission, rapid onset, and poor prognosis, it is crucial that we quickly find a cure for the many who have been affected with EVD. Zmapp has quickly become the forerunner for this cause. However, I wonder if a similar, if not greater, importance should be directed towards a different measure of “treating” EVD—a preventative one. Vaccines are a somewhat prophylactic means of treating certain viral disease, such as hepatitis, influenza, or the common chickenpox, and with great efficacy; thus, it may be shrewd to move in a similar direction with the Ebola virus as well. EVD has proven to be very contagious and progressive, easily and quickly infecting those close contact. If there was a way to prevent the onset of this disease organically before the disease progresses to a detrimental point, control of this virus would be much more effective. One promising experimental EVD vaccine has been in the works for the past few years but has recently been pushed into clinical trials due to the emergent outbreak. Developed by GlaxoSmithKline and NIH’s National Institute of Allergy and Infectious Diseases, this cAd3 Ebola vaccine (cAd3-EBO) incorporates genetic material from two different strains of the Ebola virus and uses a benign chimpanzee adenovirus vector for clinical administration. Although the trials are still in its early stages, positive results have been reported. Researches have detected increasing production of not only anti-Ebola antibodies but also the crucial CD8 T-cells vital to any effective immunologic responses. They have also denied any serious or adverse side effects to the vaccine. This clinical trial is still in its infancy, and data from trials being carried out in Africa will be key in determining if the vaccine has a similar profile in populations most at risk from Ebola; however, this seems to be moving in a positive direction for the future of this lethal disease. It will be interesting to see the efficacy of cAd3-EBO vaccine and any long-term effects that it may hold.
The sudden outbreak of the Ebola virus caused many researchers to research a vaccine that is effective to humans. The Ebola virus replicates at an unusually high rate that overwhelms the protein synthesis apparatus of infected cells and host immune defenses. This leads the protein synthesis to eventually slowly shut down. In the study, (Inhibition of Ebola Virus Infection: Identification of Niemann-Pick C1 as the Target by Optimization of a Chemical Probe), adamantane dipeptides was identified as an inhibitor of Ebola virus (EboV) infection. EboV attach to lectins on the surface of susceptible cells and are transported to late endosomes/lysosomes (LE/LY) containing host proteins cathepsin B and Niemann-Pick C1 (NPCI) which are essential for infection. Once the EBoV glycoprotein (GP) is cleaved by cathepsin protease, the cleaved GP is a ligand for NPCI. The adamantane dipeptides by high-throughout screening and hit-to-lead optimization target the lysosome membrane protein NPCI and interfere with its function as a receptor for EboV GP. NPCI provides a promising target for antiviral therapy. The adamantane dipeptides may be similar to the drug maraviroc, which inhibits HIV infection by targeting the gp120 receptor CCR5. It is important to know the regulation of adamantane dipeptides because as seen in HIV and now in EbOV this is leading to inhibition of the diseases. A vaccination should be made to increase regulation of adamantane dipeptides so future diseases that uprise like, HIV or EboV will not cause a major outbreak.
Thus far Zmapp has been administered to several animals including guinea pigs, mice and monkeys but out of all animals tested, the monkeys seem to have had the most success with 18 infected monkeys fully recovered from the infection. It makes sense that humans were able to receive the vaccinations shortly after these studies since a primate’s immune system is very similar to a human’s.. After some research, I found it interesting that comparing humans to primates helps fight disease. For example, a way to fight AIDS is by researching primates who are resistant to HIV. Because Zmapp has been given to monkeys who were infected with Ebola, I wonder if it will be possible for researchers to study it the same way they did with AIDS in the future- by finding those primates who are resistant.
I think that the problem with current state of the Ebola vaccine is cost. One of the main requirements for any vaccine to be truly effective is to have it be of an affordable cost so the masses are able to receive the vaccine. According to this CNN Health article, there is no clear defined cost of ZMapp. Though I agree that a cocktail of monoclonal antibodies may be a good way to attack a disease such as Ebola, it may not be the best, in terms of overall effectiveness. We will not really have a disease like this under control until we are able to concoct something that is just as effective and definitely of low cost.
One of the problems of developing a novel vaccine is that the development guidelines do not really follow standard protocol and the overall ethical rules created by various organizations. In a standard clinical trial, the physician or medical staff must warn the patient that they might be receiving a placebo. In the scenario of Ebola, that kind of “receiving placebo” statement would not be tolerated by the patients and probably the community. Patients want treatment asap. This leaves us the question that are we allowed to break standard protocol to push a drug into the market? If so, what are the new limits and standards that should be set?
Zmapp has several awesome factors working for it like those mentioned in the study. My issue with Zmapp is the ease at which we can use it in Africa. The Ebola epidemic is set in area where there is not a lot of infrastructure. Electricity can be hard to find in many of the villages where Zmapp would be needed. With Zmapp being made up of monoclonal antibodies, refrigeration would be needed to keep them from denaturing. If the drug is not sturdy enough then it will not survive to be used in the patients. These problems were faced in the past with both the small pox vaccine and the polio vaccine. Smallpox in Africa during Colonial Rule They solved the problem by harvesting fresh vaccinia from an inoculated cow that they kept with them. There is already a huge lack of trust of those in the Ebola hospitals. If we bring a drug that becomes denatured this could cause more unrest. If infrastructure can not be improved then a way of stabilizing the antibodies to heat will need to be done.
I was at first skeptical about the effectiveness of Zmapp against the supportive treatments being received by Ebola survivors at Emory. However now that I’ve read about the mechanism (monoclonal antibodies binding (mAbs) to three specific epitopes on the virus) of neutralizing the virus, I now have questions about the production process utilizing tobacco plants. It seems as though Zmapp is working through a plant-human passive immunity technique, rather than giving antibodies from previous Ebola survivors to current Ebola sufferers – another idea that was pitched as an Ebola treatment. What’s amazing to me though, is how people will embrace and consume products from a transgenic, genetically modified plant that produces these mAbs, but not other GMO food and animal products. In fact, using transgenic plants for mAb production has been demonstrated in research since 1989. Specific to Zmapp, transgenic tobacco plants were created with fucosyl- and xylosyl-transferase knocked out for concern that these residues may affect the pharmacokinetics of the resulting drug (Zhang et al. 2014). With glycosylation now ideal for human use, plant production of antibodies presents the possibility of large-scale, rapid, and fine-tuned antibody production. This could come in handy for later threats of epidemics or pandemics – I only wish consumers would pay more attention to how these mAb drugs are made, and be able to relate them to transgenic techniques in general, like those used to create GMO food and animal products and byproducts.
I was at first skeptical about the effectiveness of Zmapp against the supportive treatments being received by Ebola survivors at Emory. However now that I’ve read about the mechanism (monoclonal antibodies binding (mAbs) to three specific epitopes on the virus) of neutralizing the virus, I now have questions about the production process utilizing tobacco plants. It seems as though Zmapp is working through a plant-human passive immunity technique, rather than giving antibodies from previous Ebola survivors to current Ebola sufferers – another idea that was pitched as an Ebola treatment. What’s amazing to me though, is how people will embrace and consume products from a transgenic, genetically modified plant that produces these mAbs, but not other GMO food and animal products. In fact, using transgenic plants for mAb production has been demonstrated in research since 1989. Specific to Zmapp, transgenic tobacco plants were created with fucosyl- and xylosyl-transferase knocked out for concern that these residues may affect the pharmacokinetics of the resulting drug (Zhang et al. 2014). With glycosylation now ideal for human use, plant production of antibodies presents the possibility of large-scale, rapid, and fine-tuned antibody production. This could come in handy for later threats of epidemics or pandemics – I only wish consumers would pay more attention to how these mAb drugs are made, and be able to relate them to transgenic techniques in general, like those used to create GMO food and animal products and byproducts.